Lab: Identify Weak Acid Using Titration Curve
The lab report must include; Introduction, Hypothesis (if available), Materials and Methods, Results, and Discussion. THIS IS THE LAB needs to be written in essay format
- Acid-base reactions are double-displacement reactions characterized by the formation of an ionic salt and water.
- A titration is a method of determining the properties of an unknown solution by monitoring the quantity of a solution of known concentration necessary to react with it.
- The solution with the known concentration in a titration is called the titrant. The solution being studied is called the analyte.
- A solution with a precisely known concentration is referred to as a standard solution.
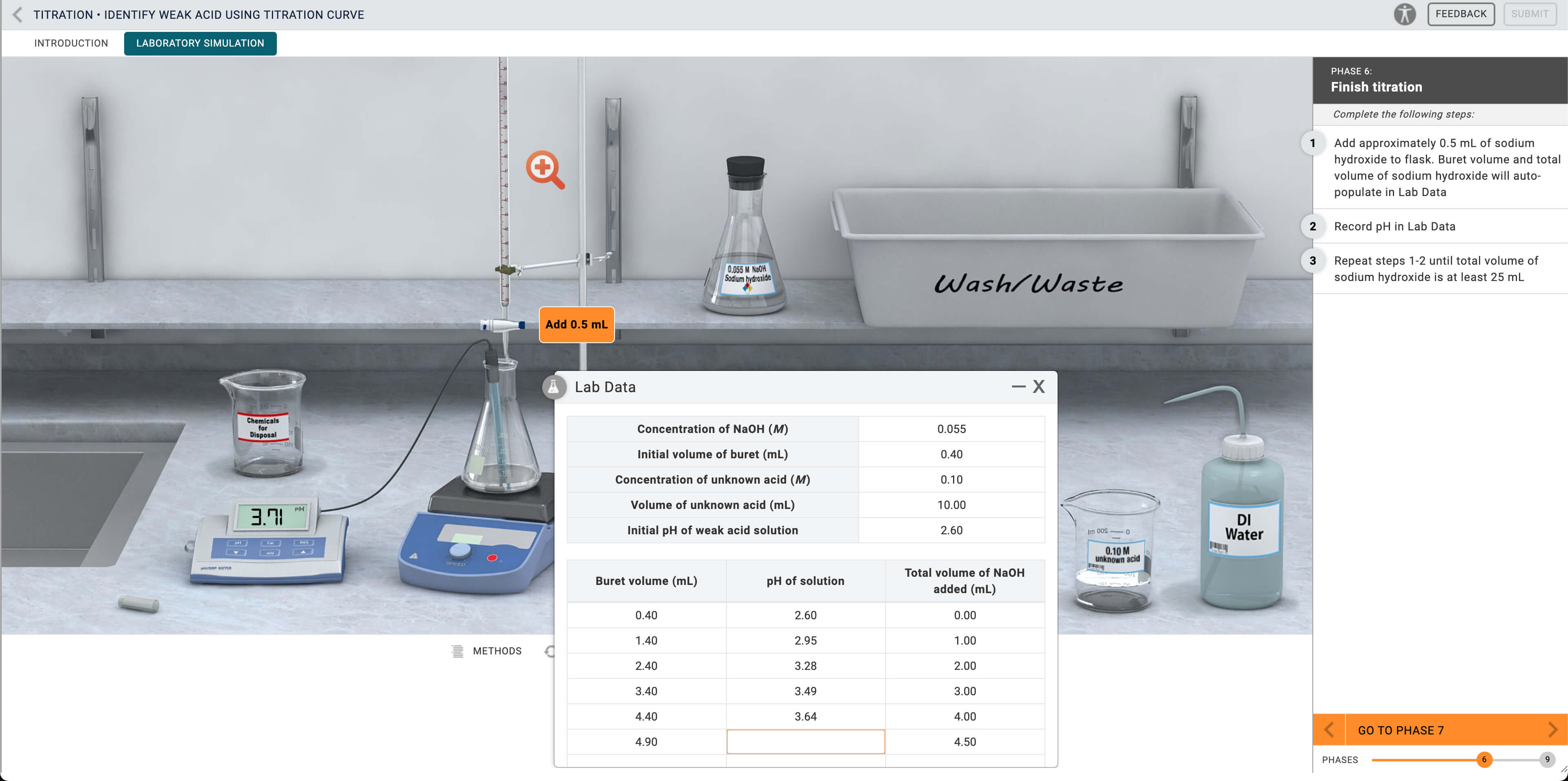
- A titration curve is a plot of pH versus volume of titrant added.
- The equivalence point in a titration is the point when the amount of titrant added is just enough to react completely with the analyte solution.
- At the half-equivalence point of a weak acid-strong base titration, the pH of the solution equals the pKa of the weak acid.
Overview
- In this simulation, you will learn about titration curves and weak acids.
- You will perform a titration where the analyte is a weak monoprotic acid and the titrant is a strong base.
- You will create a titration curve.
- You will use the titration curve to make a tentative identification of the weak acid from its pKa value.
- You will be analyzing one of the following acids:
| Weak Acid | pKa |
|---|---|
| Chloroacetic acid | 2.85 |
| Hydrofluoric acid | 3.15 |
| Formic acid | 3.77 |
| Benzoic acid | 4.19 |
| Acetic acid | 4.74 |
Scoring
In this lab simulation, your grade will be based on the following:
- Answering each question correctly
- Correctly following the outlined steps of the procedure
- Making precise and accurate measurements for your Lab Data
- Calculating precise and accurate values for your Lab Data
- Reporting your measured and calculated values with the correct number of significant figures
Before you begin
- The reaction that occurs between an unknown weak monoprotic acid and sodium hydroxide is:
NaOHâ¡(aâ¢q)+HAâ¡(aâ¢q)⟶NaAâ¡(aâ¢q)+Hâ¢A2â¢Oâ¡(l)
- where HA represents the unknown weak monoprotic acid.
- The titration curve is generated by measuring the pH after incremental additions of base to the acid. A buret is used to precisely measure the amount of base added.
- Titration of all weak acids with a strong base produces a modified S-curve. The actual shape of the curve depends on the specific acid being titrated and its pKa value.
- The key features of a titration curve are the half-equivalence point and equivalence point.

"96% of our customers have reported a 90% and above score. You might want to place an order with us."

